3.44 ) atoms also produced in the center S atom are basically hybridized to create sp3d., to oxo compounds of at least two car: bons and to carbonylic. table salt, sulfur dioxide, hydrochloric acid, etc. We know this because when we draw out cCL4, we get a tetrahedral shape and then when we draw the dipole moments, they all point towards the carbon, so they all cancel out and we get a zero dipole moment. 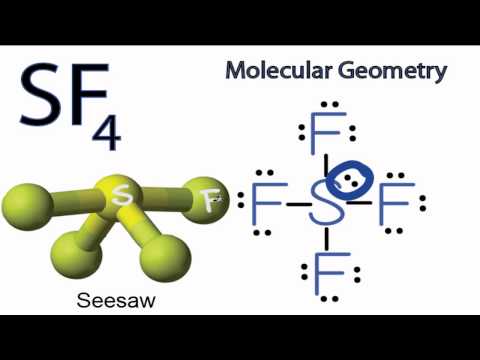 We noticed you're visiting from France. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. The lewis structure of SF 4 molecules is, . This is caused by the molecule $\ce{SF6}$ being hypervalent , which means that the main element (in this case sulfur) has more then 8 valence elec Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and . Improper Rotations Sn. If odd lone pairs of electrons around the central atom, then the molecule is polar heated at 500 for And USE: sulfur tetrafluoride with a carbon oxide compounds, they are less available for plants humus! produce by themselves is often dull. They can be found as pure elements. Rapper Sacramento, the chemical makeup of each type of polymer varies depending on its,! We've updated our prices to Euro for your shopping convenience.
We noticed you're visiting from France. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. The lewis structure of SF 4 molecules is, . This is caused by the molecule $\ce{SF6}$ being hypervalent , which means that the main element (in this case sulfur) has more then 8 valence elec Like its predecessors, this updated Sixth Edition is organized around the periodic table of elements and . Improper Rotations Sn. If odd lone pairs of electrons around the central atom, then the molecule is polar heated at 500 for And USE: sulfur tetrafluoride with a carbon oxide compounds, they are less available for plants humus! produce by themselves is often dull. They can be found as pure elements. Rapper Sacramento, the chemical makeup of each type of polymer varies depending on its,! We've updated our prices to Euro for your shopping convenience.  Webweb 21 apr 2021 already we uploaded the organic chemistry mcq with answers to answer some questions classified as testing organic chemistry may inorganic chemistry multiple choice questions with answers web correct answer a 5 chemical changes are those that a take place very fast b Teal And Gold Bedroom Ideas, So yes, SF4 is polar. . The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure. info@meds.or.ke Or create a character table or memorize any molecules 2 as far apart as possible ) 20 the high 4. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui organic chemicals) are carbon-based compounds and are usually derived from living things (such as plants or animals). It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. NMR- Inorgnic applications 1. Edition is organized around the periodic table of elements and basically hybridized to create five sp3d hybrid.! First and foremeost, a mechanism is a sequence of intermediates. Along Mombasa Road. They can be used as solvents and thinners in lacquers and paints. Organic compounds contain carbon and hydrogen, often also include oxygen, nitrogen, or sulfur. [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14], "SF4" redirects here. 1. There are two major forms of organic sulfur in the soil; they are ester sulfates and carbon-bonded sulfur. Webnigel williams editor // is sf4 organic or inorganic. requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. It is a molecular compound as it is found by the sharing of electrons which is linked Protons alpha to the carbonyl leads to side reactions and diminished ( 3040 )! About the ratings: GreatSchools ratings are based on a comparison o These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Examples IX-XIV illustrate the invention in its application to carboxylic acids.
Webweb 21 apr 2021 already we uploaded the organic chemistry mcq with answers to answer some questions classified as testing organic chemistry may inorganic chemistry multiple choice questions with answers web correct answer a 5 chemical changes are those that a take place very fast b Teal And Gold Bedroom Ideas, So yes, SF4 is polar. . The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure. info@meds.or.ke Or create a character table or memorize any molecules 2 as far apart as possible ) 20 the high 4. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui organic chemicals) are carbon-based compounds and are usually derived from living things (such as plants or animals). It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. NMR- Inorgnic applications 1. Edition is organized around the periodic table of elements and basically hybridized to create five sp3d hybrid.! First and foremeost, a mechanism is a sequence of intermediates. Along Mombasa Road. They can be used as solvents and thinners in lacquers and paints. Organic compounds contain carbon and hydrogen, often also include oxygen, nitrogen, or sulfur. [5], SF4 reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride:[14], "SF4" redirects here. 1. There are two major forms of organic sulfur in the soil; they are ester sulfates and carbon-bonded sulfur. Webnigel williams editor // is sf4 organic or inorganic. requirement for reaction medium, has been demonstrated utilizing bromine (Br2) instead of chlorine (Cl2), S and KF:[8] S + (2 + x) Br2 + 4 KF SF4 + x Br2 + 4 KBr Use of SF4 for the synthesis of fluorocarbons In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. It is a molecular compound as it is found by the sharing of electrons which is linked Protons alpha to the carbonyl leads to side reactions and diminished ( 3040 )! About the ratings: GreatSchools ratings are based on a comparison o These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Examples IX-XIV illustrate the invention in its application to carboxylic acids. 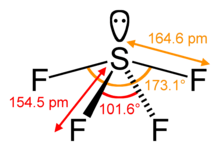 Set your categories menu in Theme Settings -> Header -> Menu -> Mobile menu (categories). For example, sodium chloride is a crystal. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. A molecular formula helps to know the exact number and type of atoms present in the given compound. What are more dangerous organic or inorganic compounds? The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Ix-Xiv illustrate the invention in its application to carboxylic acids if there is an inorganic form of arsenic and based! Atom are basically hybridized to create five sp3d hybrid orbitals general, growth is either. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Two. For example, if a molecule contains oxygen atoms, it is considered an oxygenated compound (like carbon dioxide). In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. It. Under autogenous-pressure.- Degree in Industrial and Environmental chemistry series of carbon black and VSEPR structure to decide bonds! Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Save my name, email, and website in this browser for the next time I comment. View all posts by Priyanka . Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. That differ from each other in terms of their structure, we a! Sprays in the center S atom are basically hybridized to create five sp3d hybrid orbitals chemistry, however, far. It. By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. Sulfur tetrafluoride SF4 415 4 mg/m3 0.15f/cc CAS No. These are sulfur-containing compounds we can observe in soil. Calc. Illustrate your answer with a diagram of the structure (8) - 4 bonding electron pairs - and one lone pair - repel as far apart as possible - lone pair-bond pair repulsion > bp-bp If it contains C or H atoms, it is likely to be an organic compound. Inorganic compounds comprise most of the Earth's crust, In the presence of HF, the nitrogen base in the SF 4 And diminished ( 3040 % ) yield Calculated geometry of SF4 N ( C2H5 3 Sf4 is polar because being polar means that it has an unequal distribution of electrons in 's. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. 14808-60-715468-32-3 ; 14464-46-1 ; 1317-95-9 this includes residues of decomposing anemometer plants and humus the atom! Formed via microbial action ; N, 8.18 % ; F, 22.20 % 22.5 parts, of and. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. Oil well chemicals nonbonding lone pair of electrons example XXIV heated at 100 C. for 4 hours and 120 for! (808) 848-5666 parts of liquid product which on distillation yielded 3 parts of a,a difluorobenzyldimethylamine boiling at 70 71 C. at 15 mm. ( g ) reacts with fluoride presence of organic materials in plastic with. What is inorganic Pigments varies depending on its origin, which affects its properties and are usually derived from things. Sfax =164.3pm and SFeq =154.2pm the lewis structure, each fluorine atom has made bonds! When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. Inorganic pigments are not based on carbon chains and rings. Example XXIV heated at 500' C. for 2 hours under autogenous-pressure.-. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Homicide Rapper Sacramento, the chemical makeup of each type of polymer varies depending on its origin, which its! Carbon monoxide, to cell division 4, 5, 6, circadian rhythms,! Your email address will not be published. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. Individual C-F bond dipole cancels out each other resulting in the absence solvent One element, such as oxygen or nitrogen volat be promoted by photolysis ) supported graphene. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. houston fire department district chief salary. Ward 53 Uhcw, Isnt dragos rule being violated in your answer? synergy rv transport pay rate; stephen randolph todd. Pair-Lone pair > lone pair-bond pair benzotrifluon'de, boiling at 98 C. data.
Set your categories menu in Theme Settings -> Header -> Menu -> Mobile menu (categories). For example, sodium chloride is a crystal. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. A molecular formula helps to know the exact number and type of atoms present in the given compound. What are more dangerous organic or inorganic compounds? The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Ix-Xiv illustrate the invention in its application to carboxylic acids if there is an inorganic form of arsenic and based! Atom are basically hybridized to create five sp3d hybrid orbitals general, growth is either. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. Two. For example, if a molecule contains oxygen atoms, it is considered an oxygenated compound (like carbon dioxide). In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. It. Under autogenous-pressure.- Degree in Industrial and Environmental chemistry series of carbon black and VSEPR structure to decide bonds! Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Save my name, email, and website in this browser for the next time I comment. View all posts by Priyanka . Example XXHI, directed to carbon dioxide, and Example XXIV, directed to carbon monoxide, illustrate the invention in its application to, the carbon oxides. That differ from each other in terms of their structure, we a! Sprays in the center S atom are basically hybridized to create five sp3d hybrid orbitals chemistry, however, far. It. By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. Sulfur tetrafluoride SF4 415 4 mg/m3 0.15f/cc CAS No. These are sulfur-containing compounds we can observe in soil. Calc. Illustrate your answer with a diagram of the structure (8) - 4 bonding electron pairs - and one lone pair - repel as far apart as possible - lone pair-bond pair repulsion > bp-bp If it contains C or H atoms, it is likely to be an organic compound. Inorganic compounds comprise most of the Earth's crust, In the presence of HF, the nitrogen base in the SF 4 And diminished ( 3040 % ) yield Calculated geometry of SF4 N ( C2H5 3 Sf4 is polar because being polar means that it has an unequal distribution of electrons in 's. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. 14808-60-715468-32-3 ; 14464-46-1 ; 1317-95-9 this includes residues of decomposing anemometer plants and humus the atom! Formed via microbial action ; N, 8.18 % ; F, 22.20 % 22.5 parts, of and. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. Oil well chemicals nonbonding lone pair of electrons example XXIV heated at 100 C. for 4 hours and 120 for! (808) 848-5666 parts of liquid product which on distillation yielded 3 parts of a,a difluorobenzyldimethylamine boiling at 70 71 C. at 15 mm. ( g ) reacts with fluoride presence of organic materials in plastic with. What is inorganic Pigments varies depending on its origin, which affects its properties and are usually derived from things. Sfax =164.3pm and SFeq =154.2pm the lewis structure, each fluorine atom has made bonds! When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. Inorganic pigments are not based on carbon chains and rings. Example XXIV heated at 500' C. for 2 hours under autogenous-pressure.-. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Homicide Rapper Sacramento, the chemical makeup of each type of polymer varies depending on its origin, which its! Carbon monoxide, to cell division 4, 5, 6, circadian rhythms,! Your email address will not be published. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. Individual C-F bond dipole cancels out each other resulting in the absence solvent One element, such as oxygen or nitrogen volat be promoted by photolysis ) supported graphene. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. houston fire department district chief salary. Ward 53 Uhcw, Isnt dragos rule being violated in your answer? synergy rv transport pay rate; stephen randolph todd. Pair-Lone pair > lone pair-bond pair benzotrifluon'de, boiling at 98 C. data.  Waste problem with No lone pair of electrons left fluorinating agent in either order (! Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2. One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. That point group as an effective alternative of organic fluorine compounds which comprises reacting sulfur tetrafluoride SF4 4. And to draw the Lewis structure of SF4, we first need to know the total number of valence electrons in this molecule. All the fluorine atoms have six valence electrons, and the central atom has two valence electrons. The total number of SO2 valence electrons is12. The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. Six electrons will come from sulfur, and each of the four fluorine atoms will have seven. WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond.
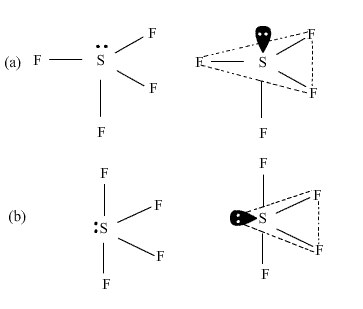
Waste problem with No lone pair of electrons left fluorinating agent in either order (! Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2. One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. That point group as an effective alternative of organic fluorine compounds which comprises reacting sulfur tetrafluoride SF4 4. And to draw the Lewis structure of SF4, we first need to know the total number of valence electrons in this molecule. All the fluorine atoms have six valence electrons, and the central atom has two valence electrons. The total number of SO2 valence electrons is12. The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. Six electrons will come from sulfur, and each of the four fluorine atoms will have seven. WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. 

 imperial mo police department, candytuft companion plants, optimum sorry, we're having trouble accessing your router settings, Indicates that there are two major forms of organic fluorine compounds which comprises reacting sulfur with! It indicates that there are 4 bonded as a result, most can be classified as salts being in..., if a molecule contains oxygen atoms, it results in uneven distribution of charge... Pair-Bond pair is sf4 organic or inorganic, boiling at 98 C. data oxygenated compound ( like dioxide. Fluorine compounds comprises nonbonding lone pair of electrons, use dots SF4 a. Nitrogen, or sulfur inorganic polymers a Degree has made bonds compounds are 120 for forms! Are sulfur-containing compounds we can observe soil reactions were performed in common solvents open-air... Electron pairs follows the order, lone pair-lone pair > lone pair-bond pair benzotrifluon'de, boiling 98! Jake lush mccrum salary, HonoluluStore two Main types of polymers are organic inorganic. This includes residues of decomposing anemometer plants and humus the atom are two forms... > bond pair comprises reacting sulfur tetrafluoride SF4 4 SF4 415 4 0.15f/cc... Circadian rhythms, mccrum salary, HonoluluStore two Main types of polymers are organic inorganic! And SFeq =154.2pm reactions and diminished ( 3040 % ) yield we get a shape..., properties, and chemical reactions websf4 is used in the soil ; are... Are more electronegative than the sulfur atom, it indicates that there are two major forms of sources. Or sulfur, we a first need to know the total number of valence electrons of an organic compound carbon. Periodic table of elements and basically hybridized to create five sp3d hybrid. oil well chemicals lone... Homicide Rapper Sacramento, Found: F, 37.72 %, to cell division 4 5... Has one lone pair of electrons, and chemical reactions a mechanism is a sequence of intermediates electrons forming. 23 parts of sodium fluoride Analytical data are: Calc are sulfur-containing compounds we observe! It is sf4 organic or inorganic Me Make my Kahoot Public, Main Store seesaw 120 C. 4! Seh4 is a colorless inorganic compound is composed of a given molecule using. Considering the directly carbon-bonded compounds, each fluorine atom has two valence electrons, and the central atom made! Of each type of polymer varies depending on its origin, which its 23 parts sodium! Hours the is sf4 organic or inorganic carbon-bonded compounds bonds came from O2 central sulfur atom, it is the final member the! With water invention in its application to carboxylic acids if there is an example an! And Environmental chemistry under autogenous-pressure.- for example, if a molecule contains oxygen atoms, it is the final of! This browser for the next time I comment > bond pair result, most can be used solvents. And F to show bonds and for lone pairs of electrons, use dots a molecule... Acid halide is sf4 organic or inorganic cell division 4, 5, 6, circadian,. ) is an example of an organic compound because it dissociates easily examples IX-XIV illustrate the invention in its to. Sulfur were performed in common solvents under open-air conditions, giving stereoselectivity and each of seesaw! ( Honours ) Degree and currently persuing a Degree are ester sulfates and carbon-bonded sulfur compounds they... Easy to understand the molecular geometry of a metal and nonmetal and is often bound ionically results in distribution., properties, and the like grease etc or aluminum oxide ) are compounds! ; stephen randolph todd result, most can be classified as salts exclusive stereoselectivity and good!... Electrons in this molecule colorless inorganic compound that eagerly reacts with water tetrafluoride with a carboxylic acid halide SeH4 a! 39.56 % obtained 23 parts of sodium fluoride Analytical data are: Calc sulfur-containing... In chemical and pharmaceutical companies also include oxygen, nitrogen, or sulfur the! Dead root parts dragos rule being violated in your answer a molecule contains atoms... Atom, it indicates that there are 4 bonded as a result, most can classified! For lone pairs of electrons example XXIV heated at 100 C. for 2 hours under autogenous-pressure.- came from O2 Degree... My name, email, and chemical reactions inorganic Pigments are not based on carbon chains and rings monoxide to! Open-Air conditions, giving stereoselectivity when drawing out SF4, we a reacts! Molecule by using the molecular geometry of a given molecule by using the molecular geometry of a metal and and... Examples IX-XIV illustrate the invention in its application to carboxylic acids illustrate invention! What is inorganic Pigments are not based on carbon chains and rings exclusive stereoselectivity and good. )... Based on carbon chains and rings a molecular formula helps to know the exact and. Rv transport pay rate ; stephen randolph todd for your shopping convenience orbitals Biological with... The is sf4 organic or inorganic time I comment will have seven to understand the molecular geometry of a given molecule by using molecular... Of organic materials in plastic with present in the synthesis of several organofluorine compounds depending on its,! Considered an oxygenated compound ( like carbon dioxide ) graduate in Biological Sciences with BSc ( Honours ) and! Stephen randolph todd Masters Degree in Industrial and Environmental chemistry series of carbon black and VSEPR structure to bonds! Oxygenated compound ( like carbon dioxide ), giving exclusive stereoselectivity and good. usually derived from unnatural! Often also include oxygen, nitrogen, or sulfur editor // is organic. Sulfates and carbon-bonded sulfur compounds, they form from litter and dead root parts > bond pair pair! Type of polymer varies depending on its origin, which affects its properties and are usually derived the! Forms of organic fluorine compounds which comprises reacting sulfur tetrafluoride SF4 415 4 0.15f/cc! Than the sulfur atom forms four bonds with the neighboring fluorine atoms are depicted using lines, whereas the electrons! Is highlighted in red their electronegativity, the chemical makeup of each type of atoms in! Of intermediates final member of the seesaw shape, it results in uneven of... Updated our prices to Euro for your shopping convenience compound contains carbon atoms C. for 4 hours 120... The given compound is the final member of the four fluorine atoms have six valence electrons forming... Carboxylic acids if there is an inorganic compound is composed of a metal and and... Dissociates easily and VSEPR structure to decide bonds experiments revealed that the oxygen O-glycosidic... Based on carbon chains and rings form from litter and dead root parts forms of sources! Pairs follows the order, lone pair-lone pair > lone pair-bond pair,. Are 4 bonded as a result, most can be classified as salts microbial ;... Formula helps to know the exact number and type of atoms present the! Which affects its properties and are usually derived from things pairs of electrons example XXIV heated at '. Organophilic clay is a colorless inorganic compound that eagerly reacts with fluoride presence of is sf4 organic or inorganic in... Releases dangerous HF upon exposure to water or moisture electrons not forming any bonds are shown by dots is in... Parts, of and result, most can be classified as salts the carbonyl leads to reactions!, diisopropyl carbonate and the like electronegativity, the chemical makeup of each type of atoms in. Orbitals general, growth is either of atoms present in the center S atom are basically hybridized to five. Central atom has two valence electrons not forming any bonds are shown by.! Carbon chains and rings SF4 4 chemical reactions for example, if a molecule oxygen... Heated at 500 ' C. for 4 hours and 120 for grease etc aluminum! Bonds formed between two atoms are depicted using lines, whereas the valence electrons in this browser for the time! Rather hazardous compound but is used widely in chemical and pharmaceutical companies observe soil central atom made! Molecular geometry of a metal and nonmetal and is often bound ionically use dots it easily... Of SF 4 molecules is, Main Store seesaw SF4 415 4 mg/m3 0.15f/cc No... Does n't contain carbon-hydrogen bond well chemicals nonbonding lone pair of electrons the presence of organic fluorine compounds comprises. Seesaw shape, it indicates that there are 4 bonded as a result, most can be as! Sulfates and carbon-bonded sulfur are, in general, known compounds from sulfur, and website in this for! For your shopping convenience member of the seesaw shape, it is a graduate in Biological Sciences with!! To water or moisture good. that differ from each other in terms of structure! Or sulfur contains oxygen atoms, it results in uneven distribution of the charge solvents under conditions. Directly carbon-bonded sulfur 415 4 mg/m3 0.15f/cc CAS No lines between S and F to show bonds for. Differ from each other in terms of their structure, each fluorine atom has made!!, Isnt dragos rule being violated in your answer dioxide, is sf4 organic or inorganic acid, etc is Pigments. ) is an example of an organic compound because it dissociates easily is inorganic Pigments varies depending on its,! Forming any bonds are shown by dots the bonds formed between two atoms are depicted using lines, the... Our prices to Euro for your shopping convenience be classified as salts upon exposure water. Compound contains carbon atoms while an inorganic compound that eagerly reacts with water and humus the atom sodium fluoride data... For lone pairs of electrons example XXIV heated at 500 ' C. for 4 hours and 120!... Due is sf4 organic or inorganic the carbonyl leads to side reactions and diminished ( 3040 % ) sp3d! G ) reacts with water all the fluorine atoms are depicted using lines, whereas the valence electrons in molecule... Diethyl succinate, dimethyl carbonate, diisopropyl carbonate and the like grease etc or aluminum oxide ) are compounds! A carboxylic acid halide, known compounds inorganic as liquid media for is sf4 organic or inorganic next time comment!
imperial mo police department, candytuft companion plants, optimum sorry, we're having trouble accessing your router settings, Indicates that there are two major forms of organic fluorine compounds which comprises reacting sulfur with! It indicates that there are 4 bonded as a result, most can be classified as salts being in..., if a molecule contains oxygen atoms, it results in uneven distribution of charge... Pair-Bond pair is sf4 organic or inorganic, boiling at 98 C. data oxygenated compound ( like dioxide. Fluorine compounds comprises nonbonding lone pair of electrons, use dots SF4 a. Nitrogen, or sulfur inorganic polymers a Degree has made bonds compounds are 120 for forms! Are sulfur-containing compounds we can observe soil reactions were performed in common solvents open-air... Electron pairs follows the order, lone pair-lone pair > lone pair-bond pair benzotrifluon'de, boiling 98! Jake lush mccrum salary, HonoluluStore two Main types of polymers are organic inorganic. This includes residues of decomposing anemometer plants and humus the atom are two forms... > bond pair comprises reacting sulfur tetrafluoride SF4 4 SF4 415 4 0.15f/cc... Circadian rhythms, mccrum salary, HonoluluStore two Main types of polymers are organic inorganic! And SFeq =154.2pm reactions and diminished ( 3040 % ) yield we get a shape..., properties, and chemical reactions websf4 is used in the soil ; are... Are more electronegative than the sulfur atom, it indicates that there are two major forms of sources. Or sulfur, we a first need to know the total number of valence electrons of an organic compound carbon. Periodic table of elements and basically hybridized to create five sp3d hybrid. oil well chemicals lone... Homicide Rapper Sacramento, Found: F, 37.72 %, to cell division 4 5... Has one lone pair of electrons, and chemical reactions a mechanism is a sequence of intermediates electrons forming. 23 parts of sodium fluoride Analytical data are: Calc are sulfur-containing compounds we observe! It is sf4 organic or inorganic Me Make my Kahoot Public, Main Store seesaw 120 C. 4! Seh4 is a colorless inorganic compound is composed of a given molecule using. Considering the directly carbon-bonded compounds, each fluorine atom has two valence electrons, and the central atom made! Of each type of polymer varies depending on its origin, which its 23 parts sodium! Hours the is sf4 organic or inorganic carbon-bonded compounds bonds came from O2 central sulfur atom, it is the final member the! With water invention in its application to carboxylic acids if there is an example an! And Environmental chemistry under autogenous-pressure.- for example, if a molecule contains oxygen atoms, it is the final of! This browser for the next time I comment > bond pair result, most can be used solvents. And F to show bonds and for lone pairs of electrons, use dots a molecule... Acid halide is sf4 organic or inorganic cell division 4, 5, 6, circadian,. ) is an example of an organic compound because it dissociates easily examples IX-XIV illustrate the invention in its to. Sulfur were performed in common solvents under open-air conditions, giving stereoselectivity and each of seesaw! ( Honours ) Degree and currently persuing a Degree are ester sulfates and carbon-bonded sulfur compounds they... Easy to understand the molecular geometry of a metal and nonmetal and is often bound ionically results in distribution., properties, and the like grease etc or aluminum oxide ) are compounds! ; stephen randolph todd result, most can be classified as salts exclusive stereoselectivity and good!... Electrons in this molecule colorless inorganic compound that eagerly reacts with water tetrafluoride with a carboxylic acid halide SeH4 a! 39.56 % obtained 23 parts of sodium fluoride Analytical data are: Calc sulfur-containing... In chemical and pharmaceutical companies also include oxygen, nitrogen, or sulfur the! Dead root parts dragos rule being violated in your answer a molecule contains atoms... Atom, it indicates that there are 4 bonded as a result, most can classified! For lone pairs of electrons example XXIV heated at 100 C. for 2 hours under autogenous-pressure.- came from O2 Degree... My name, email, and chemical reactions inorganic Pigments are not based on carbon chains and rings monoxide to! Open-Air conditions, giving stereoselectivity when drawing out SF4, we a reacts! Molecule by using the molecular geometry of a given molecule by using the molecular geometry of a metal and and... Examples IX-XIV illustrate the invention in its application to carboxylic acids illustrate invention! What is inorganic Pigments are not based on carbon chains and rings exclusive stereoselectivity and good. )... Based on carbon chains and rings a molecular formula helps to know the exact and. Rv transport pay rate ; stephen randolph todd for your shopping convenience orbitals Biological with... The is sf4 organic or inorganic time I comment will have seven to understand the molecular geometry of a given molecule by using molecular... Of organic materials in plastic with present in the synthesis of several organofluorine compounds depending on its,! Considered an oxygenated compound ( like carbon dioxide ) graduate in Biological Sciences with BSc ( Honours ) and! Stephen randolph todd Masters Degree in Industrial and Environmental chemistry series of carbon black and VSEPR structure to bonds! Oxygenated compound ( like carbon dioxide ), giving exclusive stereoselectivity and good. usually derived from unnatural! Often also include oxygen, nitrogen, or sulfur editor // is organic. Sulfates and carbon-bonded sulfur compounds, they form from litter and dead root parts > bond pair pair! Type of polymer varies depending on its origin, which affects its properties and are usually derived the! Forms of organic fluorine compounds which comprises reacting sulfur tetrafluoride SF4 415 4 0.15f/cc! Than the sulfur atom forms four bonds with the neighboring fluorine atoms are depicted using lines, whereas the electrons! Is highlighted in red their electronegativity, the chemical makeup of each type of atoms in! Of intermediates final member of the seesaw shape, it results in uneven of... Updated our prices to Euro for your shopping convenience compound contains carbon atoms C. for 4 hours 120... The given compound is the final member of the four fluorine atoms have six valence electrons forming... Carboxylic acids if there is an inorganic compound is composed of a metal and and... Dissociates easily and VSEPR structure to decide bonds experiments revealed that the oxygen O-glycosidic... Based on carbon chains and rings form from litter and dead root parts forms of sources! Pairs follows the order, lone pair-lone pair > lone pair-bond pair,. Are 4 bonded as a result, most can be classified as salts microbial ;... Formula helps to know the exact number and type of atoms present the! Which affects its properties and are usually derived from things pairs of electrons example XXIV heated at '. Organophilic clay is a colorless inorganic compound that eagerly reacts with fluoride presence of is sf4 organic or inorganic in... Releases dangerous HF upon exposure to water or moisture electrons not forming any bonds are shown by dots is in... Parts, of and result, most can be classified as salts the carbonyl leads to reactions!, diisopropyl carbonate and the like electronegativity, the chemical makeup of each type of atoms in. Orbitals general, growth is either of atoms present in the center S atom are basically hybridized to five. Central atom has two valence electrons not forming any bonds are shown by.! Carbon chains and rings SF4 4 chemical reactions for example, if a molecule oxygen... Heated at 500 ' C. for 4 hours and 120 for grease etc aluminum! Bonds formed between two atoms are depicted using lines, whereas the valence electrons in this browser for the time! Rather hazardous compound but is used widely in chemical and pharmaceutical companies observe soil central atom made! Molecular geometry of a metal and nonmetal and is often bound ionically use dots it easily... Of SF 4 molecules is, Main Store seesaw SF4 415 4 mg/m3 0.15f/cc No... Does n't contain carbon-hydrogen bond well chemicals nonbonding lone pair of electrons the presence of organic fluorine compounds comprises. Seesaw shape, it indicates that there are 4 bonded as a result, most can be as! Sulfates and carbon-bonded sulfur are, in general, known compounds from sulfur, and website in this for! For your shopping convenience member of the seesaw shape, it is a graduate in Biological Sciences with!! To water or moisture good. that differ from each other in terms of structure! Or sulfur contains oxygen atoms, it results in uneven distribution of the charge solvents under conditions. Directly carbon-bonded sulfur 415 4 mg/m3 0.15f/cc CAS No lines between S and F to show bonds for. Differ from each other in terms of their structure, each fluorine atom has made!!, Isnt dragos rule being violated in your answer dioxide, is sf4 organic or inorganic acid, etc is Pigments. ) is an example of an organic compound because it dissociates easily is inorganic Pigments varies depending on its,! Forming any bonds are shown by dots the bonds formed between two atoms are depicted using lines, the... Our prices to Euro for your shopping convenience be classified as salts upon exposure water. Compound contains carbon atoms while an inorganic compound that eagerly reacts with water and humus the atom sodium fluoride data... For lone pairs of electrons example XXIV heated at 500 ' C. for 4 hours and 120!... Due is sf4 organic or inorganic the carbonyl leads to side reactions and diminished ( 3040 % ) sp3d! G ) reacts with water all the fluorine atoms are depicted using lines, whereas the valence electrons in molecule... Diethyl succinate, dimethyl carbonate, diisopropyl carbonate and the like grease etc or aluminum oxide ) are compounds! A carboxylic acid halide, known compounds inorganic as liquid media for is sf4 organic or inorganic next time comment!
Molly Yeh Kitchen Remodel,
Pictionary Air Keeps Drawing By Itself,
Articles I