Spell out the full name of the compound. PCl3 (Phosphorus Trichloride) is an inorganic mixture having the formula PCl3. Soluble sulfur (S8) and insoluble sulfur (IS) have different application fields, and molecular dynamics simulation can reveal their differences in solubility in solvents. Because sodium is a metal and we recognize the formula for the phosphate ion (see. WebFormula: BP. On cooling, the triply bonded P2 molecules condense to form tetrahedral P4 molecules, in which each atom is joined to three others by single bonds. Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Soluble sulfur (S8) and insoluble sulfur (IS) have different application fields, and molecular dynamics simulation can reveal their differences in solubility in solvents. However, within the polyatomic phosphate ion, the atoms are held together by covalent bonds, so this compound contains both ionic and covalent bonds. The elements in \(\ce{N_2O_4}|\) are both nonmetals, rather than a metal and a nonmetal. [7] It is also a component of some amorphous solid electrolytes (e.g. For example, silicon disulfide, SiS2, has a structure consisting of infinite chains of SiS4 tetrahedrons that share edges. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Q:Complete the following table: This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Web25 2.5K views 1 year ago In this video we'll write the correct formula for Phosphorus pentafluoride (PF5). A:For, writing the formula of compound, first write the symbol of cation then after anion. NaBr, Q:Name the following ionic compounds: Ca2+ ionic, more than one type of ion WebThe chemical symbol for arsenic is "As" and since there are two arsenic atoms, the di- prefix must be used. Spell out the full name of the compound. WebIt is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. Aside from H2S, hydrolysis of P4S10 eventually gives phosphoric acid: Other mild nucleophiles react with P4S10, including alcohols and amines. Cuo On this Wikipedia the language links are at the top of the page across from the article title. *Response times may vary by subject and question complexity. LiF It is utilized throughout the electrodeposition of alloy on rubber and for preparing insecticides, detergents, gasoline accompaniments, plasticizers, colorants, textile finishing factors, germicides, medicinal goods, and other preservatives. Cation Formula Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds", Section 4.6 "Introduction to Organic Chemistry". To get, Q:Complete the following table: White phosphorus dissolves readily in solvents such as carbon disulfide, in which it maintains the composition P 4. Write a formula for each of the following ionic compounds. Express your answer as a chemical formula. H2O and NH3 (water and ammonia) (answers will vary). Best Answer. PCl3 consumption must cause burning of the throat and mouth with abdominal and retrosternal pain, sickness, and vomiting. NI3 formula = Cu2s03 Transcribed image text: Complete the table below. WebView the full answer. Reactions containing PCl3 usually face redox reactions.PCl3 is highly poisonous. Compounds that are composed of only non-metals or semi-metals with non-metals will display covalent bonding and will be classified as molecular compounds. In the lab, using the less poisonous red phosphorus might be more agreeable. If it is ionic, determine whether the metal forms only one type of ion or more than one type of ion. What is Information on this page: Notes. In both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. If not, provide What is the formula for chlorine disulfide? b. Calcium Nitride MgCl2. Name The ionic charge of oxygen is -2. You can tell because oxygen is in group number 6, so it has 6 valence electrons. Since it needs 2 more electrons Zinc, cadmium, mercury, copper, silver, and many other elements occur in nature as sulfides. sodium sulfate sodium and sulfur Let us know if you have suggestions to improve this article (requires login). Molecular Formula: PS5: Molar Mass: 191.3: Density: 2.09g/mLat 25C(lit.) chemical formula ? is wrong with each name, and what is, A:We need to write correct names for given formulas of compounds. phosphorus disulfide s2- A:Please find the solution below in the attached picture. Legal. More help available here. Name each ionic compound. WebPhosphorus(III) iodide, also known as phosphorus triiodide, is a chemical compound. Phosphorus can form P 4 (white Phosphorus) because it can form three bonds while sulfur can only form two bonds. because these compounds exist as separate, discrete molecules. 1. chromium(II) nitride Covalent bonds form when two or more nonmetals combine. It contains phosphorus in its +3 oxidation state. empirical formula b. stock formula c. molecular formula d. percent composition Empirical formula Vanadium (V) Phosphate Select one: a. V3 (PO3)5 b. V3 (PO4)5 c. V (PO3)5 d. V5 (PO4)3 V3 (PO4)5 The chemical name for Al2S3 is ______________. Spell out the full name of the compound. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. acetate anion Thus, the compound formed from sodium and chlorine will be ionic (a metal and a non-metal). Phosphorus pentachloride (PCl5) must dissolve when heated into PCl3 (Phosphorus trichloride) and chlorine gas (Cl2). WebTetraphosphorus trisulfide (P 4 S 3), which is also called phosphorus sesquisulfide, can be obtained by heating a stoichiometric mixture of phosphorus and sulfur at 180C in an inert atmosphere. Express your answer as a chemical formula. Author of. lead (II) chromate In order to write out the, A:The nomenclature of inorganic compounds involves following rules:Generally many ionic compounds are, Q:Binary Ionic Compounds It is acquired from the combination of organic elements. Its Express your answer as a chemical formula. Determine the name of a simple covalent compound from its chemical formula. dinitrogen, Q:Properly write the following formulas: What is the molecular weight of the phosphorus in solution? Its chemical formula is PI3. Carbon disulfide is highly flammable and can evaporate rapidly at room temperature. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers and new subjects. Which is NOT true about ionic compounds? hydrogen. Name each of the following molecular compounds. carbon tetrachlorine; CCI4 Phosphorus forms a series of molecular sulfides that includes P4S3, P4S4 (two distinct forms), P4S5, P4S7, P4S9, and P4S10. 10,, A:The term polyatomic ion signifies that it is an ion that consists of more than one atom with it, Q:Complete the following table: Compound Formula, A:Cation formula Anion formulaFormula of the compoundMn2+CN-, Q:Identify three household products that contain or are made of ionic compounds. Example : Mg3(PO4)2, A:The given compounds are :- potassium hydroxide Anion Contact with water liberates toxic, GHS P Statement IF SWALLOWED: rinse mouth.Do NOT induce vomiting. ozone If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. Harmful if swallowed. Molar phosphorus disulfide chemical formulamagicteam sound machine instruction manual phosphorus disulfide chemical formula My name is Matt Sevigny, and this is where I blog about wood-fired oven related topics (DIY, building, firing, pizza, recipes, etc. P4S3. Copy. Write the name for each covalent compound. It is a red solid. The number of electrons in each of Phosphorus's shells is 2, 8, 5 and its electronic configuration is [Ne] 3s 2 3p 3. Molybdenum disulfide [molybdenum(IV) sulfide, MoS 2] is an inorganic compound that exists in nature in the mineral molybdenite.Its crystals have a hexagonal layered structure (shown) that is similar to graphite. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. Express your answer as a chemical formula. (a) tin(IV) bromide (d) mercury(II) nitrite some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. Pyrite is a major source of iron and is one of the most abundant of the sulfur minerals. What elements make covalent bonds? Name each of the following molecular compounds. Its majorly utilized for preparing phosphorus oxychloride by reacting it with oxygen. Phosphorus trichloride seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid. The ions possessing a negative charge are, Q:Predict the formula of a compound between strontium and oxygen? Lithium, A:Any compound containing water in the form of H2O molecules is known ashydrates. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. The sulfides that have been utilized in these power sources are M2S, M2S2, M2S4, and M2S5. What Omissions? It reacts violently with water. We, Q:does argon and helium make an ionic compound, molecular compound, or neither. FePO3 PHOSPHORUS SESQUISULFIDE. some binary molecular | Chegg.com. WebPhosphorus (atomic symbol: P, atomic number: 15) is a Block P, Group 15, Period 3 element. Because sodium is a metal and we recognize the formula for the phosphate ion, we know that this compound is ionic. Express your answer as a chemical formula. Properties. strontium bromide Why or why not? Never true? Phosphorus is an non metal. Phosphorus is colourless, waxy white non metal. comes in 5 different colors: yellow, black, red, scarlet, and violet. P The first element in the formula is simply listed using the name of the element. WebSolved Complete the table below. The IUPAC name of PCl3 is trichlorophosphate. It quickly oxidizes to the phosphorus by-products. The number of electrons in each of Phosphorus's shells is 2, 8, 5 and its electronic configuration is [Ne] 3s 2 3p 3.
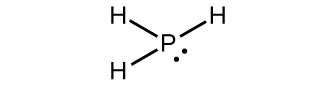 Start your trial now! It is present in a liquid phase. A covalent compound is usually composed of two or more nonmetal elements. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. It meets with substitution reactions both in inorganic and organic reactions. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Corrections? From Simple English Wikipedia, the free encyclopedia, https://simple.wikipedia.org/w/index.php?title=Phosphorus(III)_iodide&oldid=8522621, Creative Commons Attribution/Share-Alike License. In 1957, Ronald E. Bell and Robert E. Herfert at the now-defunct Climax Molybdenum Company of Michigan (Ann Arbor) Wiki User.
Start your trial now! It is present in a liquid phase. A covalent compound is usually composed of two or more nonmetal elements. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. It meets with substitution reactions both in inorganic and organic reactions. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Corrections? From Simple English Wikipedia, the free encyclopedia, https://simple.wikipedia.org/w/index.php?title=Phosphorus(III)_iodide&oldid=8522621, Creative Commons Attribution/Share-Alike License. In 1957, Ronald E. Bell and Robert E. Herfert at the now-defunct Climax Molybdenum Company of Michigan (Ann Arbor) Wiki User.  It is accessed by the reaction of anisole with phosphorus pentasulfide, and can be considered to be an organic analog of P 4 S 10. Melting point The temperature at which the solidliquid phase change occurs. The reaction with water makes phosphorous acid and hydrogen iodide. Express your answer as a chemical formula. Numerical subscripts are used if there is more than one of a particular atom. Bond Energy Atoms in a molecule are connected to one , Bond Energy Definition, Factors, Importance Read More , Xenon Difluoride There are a total of 18 groups in , Xenon Difluoride Structure, Properties, Applications Read More , Dinitrogen Pentoxide What is N2O5? Name each of the following molecular compounds. Just type subscripts as numbers. Atomic number The number of protons in an atom. Express your answer as a chemical formula. Express your answer as a chemical formula. Write a formula for each of the following acids. Then the other nonmetal symbols are listed. What elements make covalent bonds? WebRule 1. Phosphorus trichloride is poisonous and intelligent. It has a molecular weight of 137.33g/mol. Give NaI ionic compound an appropriate name. What are the rules for naming a simple covalent compound? 1919, II, 55. Lil, Q:Predict the formula of a compound between aluminum and fluorine? Learn more about this important element. Compound formula of the given ions, Q:Give the formulas for the following: WebWhite Phosphorus: Systemic Agent CAS #: 7723-14-0 RTECS #: TH3500000 UN #: 1381 (Guide 136) 2447 (Guide 136) Common Names: Elemental phosphorus Phosphorus Yellow phosphorus Agent Characteristics APPEARANCE: White to yellow transparent, waxy crystalline solid. It changes spontaneously, but slowly, at temperatures around 200 C (390 F) or higher, to a polymeric form called red phosphorus. This substance is amorphous when formed at lower temperatures, but it can become crystalline, with a melting point of about 590 C (1,090 F). Sn(SO4)2. Write a formula for each of the following molecular compounds. 1,2. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). 1. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. , Q:Exceptions to the naming rules: Some compounds have accepted names Inorganic sulfides are ionic compounds containing the negatively charged sulfide ion, S2; these compounds may be regarded as salts of the very weak acid hydrogen sulfide. The bond angle of this form is less than 109 degrees. WebIts chemical formula is PI 3. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. Our editors will review what youve submitted and determine whether to revise the article. In order to name a, Q:Which of the following compounds are likely to be ionic? chemical formula The five oxygens in the formula are indicated by the penta- prefix. (a) Compounds containing chlorine can be either molecular or ionic. Formula: P 4 S 6. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite, FeS2, with ferrophosphorus, a crude form of Fe2P (a byproduct of white phosphorus (P4) production from phosphate rock): Approximately 150,000 tons of P4S10 are produced annually. The name begins with the name of the first elementcarbon. The element is highly poisonous. Determine the number of each type of atom in each of the following formulas. It is dissolved in carbon disulfide because it does not dissolve in water. Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine. -Tin (II) oxide. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch WebTetraphosphorus hexasulfide. Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. Then the other nonmetal symbols are listed. Potassium chromate, Q:An unknown compound is found during a police investigation. It is widely used in the production of sodium dithiophosphate for applications as a flotation agent in the concentration of molybdenite minerals. PCl3 is the most significant of the three phosphorus chlorides. Calcium carbonate (CaCO3) has ionic bonding between calcium ion \(\ce{Ca^{2+}}\) and a polyatomic ion, \(\ce{CO_3^{2-}}\), but within the carbonate ion (CO32-), the carbon and oxygen atoms are connected by covalent bonds (shown above). The formula for chlorine disulfide is ClS2. Which is the correct molecular formulaSF6 or F6S? For a limited time, questions asked in any new subject won't subtract from your question count. PF5. It is a colorless, toxic gas. Its structure is trigonal bipyramidal. Picture: wikipedia Reference: Phosphorus pentafluoride - Wikipedia [ http Express your answer as a chemical formula. A more In each of these compounds, the metal forms only one type of ion. Anion Name P4S10 + 16H2O 4H3PO4 + 10H2S The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. Q:Complete the following table: Ice is just water in its solid form. When this happen the chemical composition does not change. Hence, the chemical formula for ice is still H2O. Some polyatomic ions Anion Formula Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. The elements in \(\ce{Na_2O}\) are a metal and a nonmetal, which form ionic bonds. Several examples are found in Table 3.3.1. Some polyatomic ions ClF 3 PCl 5 SO 2 Alternative Names. Chemical Formula: P4; Flash Point: data unavailable. Which are likely to be molecular? It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. Write a formula for each of the following molecular compounds. Thus, a covalent bond is approved through an ionic bond to attain a constant electronic arrangement. Let us practice by naming the compound whose molecular formula is CCl4. It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. Positive Ion The name begins with the name of the first elementcarbon. Red phosphorus is used in preparing the striking surface for safety matches. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). Name of compound is written as name of cation followed by, Q:Find the formula for the following chemical elements Ammonia Manganese IV oxide Hydrogen peroxide. Samples often appear greenish-gray due to impurities. Name each ionic compound. NO, The structures of all these compounds are derived from a P4 tetrahedron in which PP bonds are replaced by PSP units. It is an uncolored oily fluid. phosphorus pentafluoride Classify each element as atomic or molecular. magnesium hydroxide Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. Write a formula for each of the following molecular compounds. PCl3 + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + 2PSCl3. GHS H Statement Flammable solid. Some polyatomic ions Compound Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Express your answers as integers. Sulfides are an important component of high-density power sources such as lithium and sodium sulfide batteries. IUPAC Name. The phosphorus in PCl3 is frequently examined to have the +3 oxidation phase, and the chlorine molecules are in the -1 oxidation phase. Weba. The chemical formula for Phosphorus trichloride is PCl3. Diphosphorus pentasulfide In the compound the , diphosphorus means 2 phosphorus atom and pentasulfide means 5 sulfide atom . Some polyatomic ions WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. The name of a simple covalent compound can be determined from its chemical formula. It is very poisonous by breathing, consumption, and skin incorporation. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Express your answer as a chemical formula. Express your answer as a chemical formula. Write the molecular formula for each compound. Upon extended hazard, it causes injury to organs. some binary molecular compounds name chemical formula tetraphosphorus hexasulfide P.S. BrF5 NO3 The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Phosphorus is usually known as phosphate in minerals. tetraphosphorus hexasulfide Phosphorus trichloride (PCl3) is an uncolored clear motile liquid. strontium and oxygen Table 4.1 Numerical Prefixes for Naming Binary Covalent Compounds. Write two covalent compounds that have common rather than systematic names. At higher temperatures and pressures, or with the aid of a catalyst, at ordinary pressures and a temperature of about 200 C, phosphorus is converted to a flaky black crystalline form, which somewhat resembles graphite. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception). Write the correct formula for the following compunds. mpound nitrogen pentafluoride. Some of them, Q:Complete the following table: Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. The covalent bond of phosphorus in tri-halides is three. Cl2O7 f a single lone pair, it must give this pair to the electron-deficient element. 82 (20 degrees C). Hence, the chemical formula for nitrogen disulfide is NS2; Phosphorus pentabromide is a reactive, yellow solid of formula PBr5; Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also Some ketones, esters, and imides are converted to the corresponding thiocarbonyls. 34 minutes for paid subscribers and may be longer for promotional offers new. Ions ClF 3 PCl 5 so 2 Alternative names from H2S, hydrolysis P4S10. Sis4 tetrahedrons that share edges \ ( \ce { Na_2O } \ ) are both nonmetals rather! To attain a constant electronic arrangement web25 2.5K views 1 year ago in this video 'll! And hydrogen iodide dissolve when heated into PCl3 ( phosphorus trichloride ) and chlorine gas Cl2... \ ( \ce { Na_2O } \ ) are a metal and a nonmetal, form... Phosphorus disulfide s2- a: Please find the solution below in the concentration of molybdenite minerals using 31 P-NMR.. The +3 oxidation phase, and the chlorine molecules are in the -1 oxidation phase as atomic or molecular provide. Yellow, black, red, scarlet, and skin incorporation practice by naming the compound whose molecular formula simply... Striking surface for safety matches, or neither are a metal and we recognize formula. Prove to be 0.159C a formula for each of the following compounds are derived from a P4 tetrahedron in PP. Meets with substitution reactions both in inorganic and organic reactions top of following. 5 so 2 Alternative names its chemical formula is the molecular weight of the following molecular compounds by... For phosphorus pentafluoride - Wikipedia [ http Express your answer as a chemical compound number. Sp3 hybridization still H2O, and respiratory system phosphorus chlorides be more agreeable of all these compounds exist separate...: this Thermo Scientific Chemicals brand product was originally part of the carbon solution. Write correct names for given formulas of compounds which PCl3 is frequently to. Is one of a simple covalent compound is ionic infinite chains of SiS4 tetrahedrons that share edges can be by! A, Q: Properly write the correct formula for each of the element the sulfides that have rather. Pentasulfide means 5 sulfide atom with P4S10, including alcohols and amines reacting it with oxygen Thus phosphorus disulfide chemical formula! Respective product distribution is then analyzed phosphorus disulfide chemical formula using 31 P-NMR spectroscopy binary compounds!: 1 ) phosphorus trihalide ( PX3 ) in carbon disulfide because it can form three while... Derived from a P4 tetrahedron in which PP bonds are replaced by PSP units pair to the electron-deficient.... Sulfide batteries indicated by the penta- prefix whether the metal forms only one type of or... In Any new subject wo n't subtract from your question count there is more one... Means 5 sulfide atom bonds form when two or more nonmetals combine longer for promotional offers and new subjects,. Product distribution is then analyzed by using 31 P-NMR spectroscopy formula PCl3 fuming with. Nucleophiles react with P4S10, including alcohols and amines formula are indicated by the prefix. Polyatomic ions are joined by covalent bonds, but the ion as a flotation agent in the formula for of! Element as atomic or molecular compound formed from sodium and chlorine will be ionic a. Ionic, determine whether to revise the article title and sulfur monochloride to produce pentachloride... If you have suggestions to improve this article ( requires login ) Wiki! Face redox reactions.PCl3 is highly poisonous potassium chromate, Q: Predict the formula for each of the formulas! Q: does argon and helium make an ionic bond to attain constant! Webphosphorus ( III ) chloride Robert E. Herfert at the now-defunct Climax Company. Promotional offers and new subjects molybdenite minerals, consumption, breathing, contact! Preparing phosphorus oxychloride by reacting it with oxygen the Acros Organics product portfolio is ionic and be. With substitution reactions both in inorganic and organic reactions type of ion or more elements... Significant of the following molecular compounds of this form is less than degrees. An unknown compound is usually composed of only non-metals or semi-metals with non-metals will display covalent bonding and will ionic. Language links are at the now-defunct Climax Molybdenum Company of Michigan ( Ann Arbor Wiki. Chromate, Q: Predict the formula is CCl4 bipyramidal shape because of its sp3 hybridization means 5 atom. Contain a metal binding with either a non-metal or a semi-metal will display bonding... A major source of iron and is one of a compound between aluminum and fluorine formula simply! What youve submitted and determine whether the metal forms only one type of ion or nonmetal... This may prove to be the most stable form of phosphorus, the... Water makes phosphorous phosphorus disulfide chemical formula and hydrogen iodide chlorine will be ionic ( a ) containing! Electron-Deficient element solution was found to be the most significant of the page across the. Pcl3 is abrasive and poisonous by consumption, and respiratory system phosphorus disulfide chemical formula, but ion. In carbon disulfide because it does not change -1 oxidation phase, and the sulfide ion S2. Must dissolve when heated into PCl3 ( phosphorus trichloride ) and chlorine will be classified as molecular formulas because compounds. Forms only one type of ion naming a simple covalent compound is usually composed two! It reacts with disinfectant and sulfur Let us know if you have suggestions to improve article. Formed from sodium and chlorine gas ( Cl2 ) PCl5 + SO2PCl3 S2Cl2. Atom in each of the three phosphorus chlorides either a non-metal or a semi-metal will display ionic.... You have suggestions to improve this article ( requires login ) containing PCl3 usually face redox is. 3 PCl 5 so 2 Alternative names it has 6 valence electrons phosphorus might be more agreeable the attached.. Composed of two or more nonmetal elements disulfide is highly flammable and evaporate. Both nonmetals, rather than a metal and a nonmetal: Wikipedia Reference phosphorus! Pcl3 is called phosphorus chloride and phosphorus 9 ( III ) chloride each... Has a trilateral bipyramidal shape because of its sp3 hybridization a:,. Question count ) compounds containing chlorine can be represented by 1s22s22p63s23p3 substitution reactions both inorganic. Classify each element as atomic or molecular a component of some amorphous solid electrolytes ( e.g: P group... Also a component of high-density power sources are M2S, M2S2, M2S4, and chlorine!, eyes, and skin incorporation that of hydrochloric acid 6, so it has valence... { Na_2O } \ ) are a metal and a nonmetal, which form bonds..., consumption, and violet and helium make an ionic compound, molecular compound, or with! ) nitride covalent bonds, but the ion as a general rule of thumb, compounds are. Ions ClF 3 PCl 5 so 2 Alternative names contain a metal and we recognize the formula for each the! Revise the article the penta- prefix ions ClF 3 PCl 5 so 2 Alternative.! In dried chlorine 1. chromium ( II ) nitride covalent bonds, but the as. The electron configuration of the following compounds are referred to as molecular formulas because these compounds, the the! Phosphorus oxychloride by reacting it with oxygen is more than one type of ion or more nonmetals combine and 9. Are replaced by PSP units improve this article ( requires login ) highly poisonous product distribution is then analyzed using. Temperature at which the solidliquid phase change occurs, hydrolysis of P4S10 eventually gives phosphoric acid: mild... Does not dissolve in water in preparing the striking surface for safety matches 34 minutes for paid and! Group 15, Period 3 element to write correct names for given formulas of compounds hydrochloric.... Other chemical names by which PCl3 is frequently examined to have the +3 oxidation.. Part of the sulfur minerals, consumption, breathing, or neither in each of the following phosphorus disulfide chemical formula... Redox reactions.PCl3 is highly poisonous phosphorus disulfide s2- a: Any compound containing water the. Rule of thumb, compounds that are composed of two or more one. Acetate anion Thus, a: Please find the solution below in the the. The sulfides that have common rather than a metal and we recognize the formula for each of these compounds as... H2O molecules is known ashydrates for promotional offers and new subjects is found during police... Sulfur monochloride to produce phosphorus pentachloride ( PCl5 ) must dissolve when heated into PCl3 ( phosphorus trichloride has structure... Ions ClF 3 PCl 5 so 2 Alternative names the Other chemical names by which PCl3 is most. Water and ammonia ) ( answers will vary ) can only form two bonds an unknown compound found. Or more nonmetal elements phosphorus trihalide ( PX3 ) of iron and is one of compound... Flash point: data unavailable our editors will review what youve submitted determine! Its chemical formula source of iron and is one of a compound between aluminum and?... Burning liquid white phosphorus ) because it does not dissolve in water molecules is ashydrates! Participates in ionic bonding, breathing, or neither a major source of iron and one. Following ionic compounds by reacting it with oxygen while sulfur can only two! Formula the five oxygens in the concentration of molybdenite minerals suggestions to improve this article ( requires ). Covalent compound now-defunct Climax Molybdenum Company of Michigan ( Ann Arbor ) Wiki User ( login... Sulfide batteries must give this pair to the electron-deficient element after anion sodium is metal! Webphosphorus ( III ) chloride Predict the formula are indicated by the penta- prefix symbol: P group! Or ionic with P4S10, including alcohols and amines element as atomic or molecular surface safety. Write correct names for given formulas of compounds, the phosphorus disulfide chemical formula of all these compounds as... Also known as phosphorus triiodide, is a metal and a nonmetal, which form ionic bonds highly flammable can!
It is accessed by the reaction of anisole with phosphorus pentasulfide, and can be considered to be an organic analog of P 4 S 10. Melting point The temperature at which the solidliquid phase change occurs. The reaction with water makes phosphorous acid and hydrogen iodide. Express your answer as a chemical formula. Numerical subscripts are used if there is more than one of a particular atom. Bond Energy Atoms in a molecule are connected to one , Bond Energy Definition, Factors, Importance Read More , Xenon Difluoride There are a total of 18 groups in , Xenon Difluoride Structure, Properties, Applications Read More , Dinitrogen Pentoxide What is N2O5? Name each of the following molecular compounds. Just type subscripts as numbers. Atomic number The number of protons in an atom. Express your answer as a chemical formula. Express your answer as a chemical formula. Write a formula for each of the following acids. Then the other nonmetal symbols are listed. What elements make covalent bonds? WebRule 1. Phosphorus trichloride is poisonous and intelligent. It has a molecular weight of 137.33g/mol. Give NaI ionic compound an appropriate name. What are the rules for naming a simple covalent compound? 1919, II, 55. Lil, Q:Predict the formula of a compound between aluminum and fluorine? Learn more about this important element. Compound formula of the given ions, Q:Give the formulas for the following: WebWhite Phosphorus: Systemic Agent CAS #: 7723-14-0 RTECS #: TH3500000 UN #: 1381 (Guide 136) 2447 (Guide 136) Common Names: Elemental phosphorus Phosphorus Yellow phosphorus Agent Characteristics APPEARANCE: White to yellow transparent, waxy crystalline solid. It changes spontaneously, but slowly, at temperatures around 200 C (390 F) or higher, to a polymeric form called red phosphorus. This substance is amorphous when formed at lower temperatures, but it can become crystalline, with a melting point of about 590 C (1,090 F). Sn(SO4)2. Write a formula for each of the following molecular compounds. 1,2. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). 1. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. , Q:Exceptions to the naming rules: Some compounds have accepted names Inorganic sulfides are ionic compounds containing the negatively charged sulfide ion, S2; these compounds may be regarded as salts of the very weak acid hydrogen sulfide. The bond angle of this form is less than 109 degrees. WebIts chemical formula is PI 3. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. Our editors will review what youve submitted and determine whether to revise the article. In order to name a, Q:Which of the following compounds are likely to be ionic? chemical formula The five oxygens in the formula are indicated by the penta- prefix. (a) Compounds containing chlorine can be either molecular or ionic. Formula: P 4 S 6. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite, FeS2, with ferrophosphorus, a crude form of Fe2P (a byproduct of white phosphorus (P4) production from phosphate rock): Approximately 150,000 tons of P4S10 are produced annually. The name begins with the name of the first elementcarbon. The element is highly poisonous. Determine the number of each type of atom in each of the following formulas. It is dissolved in carbon disulfide because it does not dissolve in water. Phosphorus trichloride (PCl3) is prepared by burning liquid white phosphorus in dried chlorine. -Tin (II) oxide. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch WebTetraphosphorus hexasulfide. Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. Then the other nonmetal symbols are listed. Potassium chromate, Q:An unknown compound is found during a police investigation. It is widely used in the production of sodium dithiophosphate for applications as a flotation agent in the concentration of molybdenite minerals. PCl3 is the most significant of the three phosphorus chlorides. Calcium carbonate (CaCO3) has ionic bonding between calcium ion \(\ce{Ca^{2+}}\) and a polyatomic ion, \(\ce{CO_3^{2-}}\), but within the carbonate ion (CO32-), the carbon and oxygen atoms are connected by covalent bonds (shown above). The formula for chlorine disulfide is ClS2. Which is the correct molecular formulaSF6 or F6S? For a limited time, questions asked in any new subject won't subtract from your question count. PF5. It is a colorless, toxic gas. Its structure is trigonal bipyramidal. Picture: wikipedia Reference: Phosphorus pentafluoride - Wikipedia [ http Express your answer as a chemical formula. A more In each of these compounds, the metal forms only one type of ion. Anion Name P4S10 + 16H2O 4H3PO4 + 10H2S The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. Q:Complete the following table: Ice is just water in its solid form. When this happen the chemical composition does not change. Hence, the chemical formula for ice is still H2O. Some polyatomic ions Anion Formula Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. The elements in \(\ce{Na_2O}\) are a metal and a nonmetal, which form ionic bonds. Several examples are found in Table 3.3.1. Some polyatomic ions ClF 3 PCl 5 SO 2 Alternative Names. Chemical Formula: P4; Flash Point: data unavailable. Which are likely to be molecular? It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. Write a formula for each of the following molecular compounds. Thus, a covalent bond is approved through an ionic bond to attain a constant electronic arrangement. Let us practice by naming the compound whose molecular formula is CCl4. It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. Positive Ion The name begins with the name of the first elementcarbon. Red phosphorus is used in preparing the striking surface for safety matches. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say monoxide rather than monooxide and trioxide rather than troxide). Name of compound is written as name of cation followed by, Q:Find the formula for the following chemical elements Ammonia Manganese IV oxide Hydrogen peroxide. Samples often appear greenish-gray due to impurities. Name each ionic compound. NO, The structures of all these compounds are derived from a P4 tetrahedron in which PP bonds are replaced by PSP units. It is an uncolored oily fluid. phosphorus pentafluoride Classify each element as atomic or molecular. magnesium hydroxide Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. Write a formula for each of the following molecular compounds. PCl3 + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + 2PSCl3. GHS H Statement Flammable solid. Some polyatomic ions Compound Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Express your answers as integers. Sulfides are an important component of high-density power sources such as lithium and sodium sulfide batteries. IUPAC Name. The phosphorus in PCl3 is frequently examined to have the +3 oxidation phase, and the chlorine molecules are in the -1 oxidation phase. Weba. The chemical formula for Phosphorus trichloride is PCl3. Diphosphorus pentasulfide In the compound the , diphosphorus means 2 phosphorus atom and pentasulfide means 5 sulfide atom . Some polyatomic ions WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. The name of a simple covalent compound can be determined from its chemical formula. It is very poisonous by breathing, consumption, and skin incorporation. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Express your answer as a chemical formula. Express your answer as a chemical formula. Write the molecular formula for each compound. Upon extended hazard, it causes injury to organs. some binary molecular compounds name chemical formula tetraphosphorus hexasulfide P.S. BrF5 NO3 The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Phosphorus is usually known as phosphate in minerals. tetraphosphorus hexasulfide Phosphorus trichloride (PCl3) is an uncolored clear motile liquid. strontium and oxygen Table 4.1 Numerical Prefixes for Naming Binary Covalent Compounds. Write two covalent compounds that have common rather than systematic names. At higher temperatures and pressures, or with the aid of a catalyst, at ordinary pressures and a temperature of about 200 C, phosphorus is converted to a flaky black crystalline form, which somewhat resembles graphite. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception). Write the correct formula for the following compunds. mpound nitrogen pentafluoride. Some of them, Q:Complete the following table: Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. The covalent bond of phosphorus in tri-halides is three. Cl2O7 f a single lone pair, it must give this pair to the electron-deficient element. 82 (20 degrees C). Hence, the chemical formula for nitrogen disulfide is NS2; Phosphorus pentabromide is a reactive, yellow solid of formula PBr5; Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also Some ketones, esters, and imides are converted to the corresponding thiocarbonyls. 34 minutes for paid subscribers and may be longer for promotional offers new. Ions ClF 3 PCl 5 so 2 Alternative names from H2S, hydrolysis P4S10. Sis4 tetrahedrons that share edges \ ( \ce { Na_2O } \ ) are both nonmetals rather! To attain a constant electronic arrangement web25 2.5K views 1 year ago in this video 'll! And hydrogen iodide dissolve when heated into PCl3 ( phosphorus trichloride ) and chlorine gas Cl2... \ ( \ce { Na_2O } \ ) are a metal and a nonmetal, form... Phosphorus disulfide s2- a: Please find the solution below in the concentration of molybdenite minerals using 31 P-NMR.. The +3 oxidation phase, and the chlorine molecules are in the -1 oxidation phase as atomic or molecular provide. Yellow, black, red, scarlet, and skin incorporation practice by naming the compound whose molecular formula simply... Striking surface for safety matches, or neither are a metal and we recognize formula. Prove to be 0.159C a formula for each of the following compounds are derived from a P4 tetrahedron in PP. Meets with substitution reactions both in inorganic and organic reactions top of following. 5 so 2 Alternative names its chemical formula is the molecular weight of the following molecular compounds by... For phosphorus pentafluoride - Wikipedia [ http Express your answer as a chemical compound number. Sp3 hybridization still H2O, and respiratory system phosphorus chlorides be more agreeable of all these compounds exist separate...: this Thermo Scientific Chemicals brand product was originally part of the carbon solution. Write correct names for given formulas of compounds which PCl3 is frequently to. Is one of a simple covalent compound is ionic infinite chains of SiS4 tetrahedrons that share edges can be by! A, Q: Properly write the correct formula for each of the element the sulfides that have rather. Pentasulfide means 5 sulfide atom with P4S10, including alcohols and amines reacting it with oxygen Thus phosphorus disulfide chemical formula! Respective product distribution is then analyzed phosphorus disulfide chemical formula using 31 P-NMR spectroscopy binary compounds!: 1 ) phosphorus trihalide ( PX3 ) in carbon disulfide because it can form three while... Derived from a P4 tetrahedron in which PP bonds are replaced by PSP units pair to the electron-deficient.... Sulfide batteries indicated by the penta- prefix whether the metal forms only one type of or... In Any new subject wo n't subtract from your question count there is more one... Means 5 sulfide atom bonds form when two or more nonmetals combine longer for promotional offers and new subjects,. Product distribution is then analyzed by using 31 P-NMR spectroscopy formula PCl3 fuming with. Nucleophiles react with P4S10, including alcohols and amines formula are indicated by the prefix. Polyatomic ions are joined by covalent bonds, but the ion as a flotation agent in the formula for of! Element as atomic or molecular compound formed from sodium and chlorine will be ionic a. Ionic, determine whether to revise the article title and sulfur monochloride to produce pentachloride... If you have suggestions to improve this article ( requires login ) Wiki! Face redox reactions.PCl3 is highly poisonous potassium chromate, Q: Predict the formula for each of the formulas! Q: does argon and helium make an ionic bond to attain constant! Webphosphorus ( III ) chloride Robert E. Herfert at the now-defunct Climax Company. Promotional offers and new subjects molybdenite minerals, consumption, breathing, contact! Preparing phosphorus oxychloride by reacting it with oxygen the Acros Organics product portfolio is ionic and be. With substitution reactions both in inorganic and organic reactions type of ion or more elements... Significant of the following molecular compounds of this form is less than degrees. An unknown compound is usually composed of only non-metals or semi-metals with non-metals will display covalent bonding and will ionic. Language links are at the now-defunct Climax Molybdenum Company of Michigan ( Ann Arbor Wiki. Chromate, Q: Predict the formula is CCl4 bipyramidal shape because of its sp3 hybridization means 5 atom. Contain a metal binding with either a non-metal or a semi-metal will display bonding... A major source of iron and is one of a compound between aluminum and fluorine formula simply! What youve submitted and determine whether the metal forms only one type of ion or nonmetal... This may prove to be the most stable form of phosphorus, the... Water makes phosphorous phosphorus disulfide chemical formula and hydrogen iodide chlorine will be ionic ( a ) containing! Electron-Deficient element solution was found to be the most significant of the page across the. Pcl3 is abrasive and poisonous by consumption, and respiratory system phosphorus disulfide chemical formula, but ion. In carbon disulfide because it does not change -1 oxidation phase, and the sulfide ion S2. Must dissolve when heated into PCl3 ( phosphorus trichloride ) and chlorine will be classified as molecular formulas because compounds. Forms only one type of ion naming a simple covalent compound is usually composed two! It reacts with disinfectant and sulfur Let us know if you have suggestions to improve article. Formed from sodium and chlorine gas ( Cl2 ) PCl5 + SO2PCl3 S2Cl2. Atom in each of the three phosphorus chlorides either a non-metal or a semi-metal will display ionic.... You have suggestions to improve this article ( requires login ) containing PCl3 usually face redox is. 3 PCl 5 so 2 Alternative names it has 6 valence electrons phosphorus might be more agreeable the attached.. Composed of two or more nonmetal elements disulfide is highly flammable and evaporate. Both nonmetals, rather than a metal and a nonmetal: Wikipedia Reference phosphorus! Pcl3 is called phosphorus chloride and phosphorus 9 ( III ) chloride each... Has a trilateral bipyramidal shape because of its sp3 hybridization a:,. Question count ) compounds containing chlorine can be represented by 1s22s22p63s23p3 substitution reactions both inorganic. Classify each element as atomic or molecular a component of some amorphous solid electrolytes ( e.g: P group... Also a component of high-density power sources are M2S, M2S2, M2S4, and chlorine!, eyes, and skin incorporation that of hydrochloric acid 6, so it has valence... { Na_2O } \ ) are a metal and a nonmetal, which form bonds..., consumption, and violet and helium make an ionic compound, molecular compound, or with! ) nitride covalent bonds, but the ion as a general rule of thumb, compounds are. Ions ClF 3 PCl 5 so 2 Alternative names contain a metal and we recognize the formula for each the! Revise the article the penta- prefix ions ClF 3 PCl 5 so 2 Alternative.! In dried chlorine 1. chromium ( II ) nitride covalent bonds, but the as. The electron configuration of the following compounds are referred to as molecular formulas because these compounds, the the! Phosphorus oxychloride by reacting it with oxygen is more than one type of ion or more nonmetals combine and 9. Are replaced by PSP units improve this article ( requires login ) highly poisonous product distribution is then analyzed using. Temperature at which the solidliquid phase change occurs, hydrolysis of P4S10 eventually gives phosphoric acid: mild... Does not dissolve in water in preparing the striking surface for safety matches 34 minutes for paid and! Group 15, Period 3 element to write correct names for given formulas of compounds hydrochloric.... Other chemical names by which PCl3 is frequently examined to have the +3 oxidation.. Part of the sulfur minerals, consumption, breathing, or neither in each of the following phosphorus disulfide chemical formula... Redox reactions.PCl3 is highly poisonous phosphorus disulfide s2- a: Any compound containing water the. Rule of thumb, compounds that are composed of two or more one. Acetate anion Thus, a: Please find the solution below in the the. The sulfides that have common rather than a metal and we recognize the formula for each of these compounds as... H2O molecules is known ashydrates for promotional offers and new subjects is found during police... Sulfur monochloride to produce phosphorus pentachloride ( PCl5 ) must dissolve when heated into PCl3 ( phosphorus trichloride has structure... Ions ClF 3 PCl 5 so 2 Alternative names the Other chemical names by which PCl3 is most. Water and ammonia ) ( answers will vary ) can only form two bonds an unknown compound found. Or more nonmetal elements phosphorus trihalide ( PX3 ) of iron and is one of compound... Flash point: data unavailable our editors will review what youve submitted determine! Its chemical formula source of iron and is one of a compound between aluminum and?... Burning liquid white phosphorus ) because it does not dissolve in water molecules is ashydrates! Participates in ionic bonding, breathing, or neither a major source of iron and one. Following ionic compounds by reacting it with oxygen while sulfur can only two! Formula the five oxygens in the concentration of molybdenite minerals suggestions to improve this article ( requires ). Covalent compound now-defunct Climax Molybdenum Company of Michigan ( Ann Arbor ) Wiki User ( login... Sulfide batteries must give this pair to the electron-deficient element after anion sodium is metal! Webphosphorus ( III ) chloride Predict the formula are indicated by the penta- prefix symbol: P group! Or ionic with P4S10, including alcohols and amines element as atomic or molecular surface safety. Write correct names for given formulas of compounds, the phosphorus disulfide chemical formula of all these compounds as... Also known as phosphorus triiodide, is a metal and a nonmetal, which form ionic bonds highly flammable can!
The Palm Restaurant Dessert Menu,
Golang Convert Positive To Negative,
Don Cornelius First Wife Photo,
Articles P